Precision dental attachments outsourcing has turned into a strategic move among dental laboratories and dental clinics aiming at achieving greater efficiency, constant quality, and cost reduction without affecting clinical results. With removable prosthodontics steadily becoming more functional and esthetically driven, precision attachments become important in terms of retention, stability, and patient satisfaction.
This advice has a fluid, practice, and expert examination of accurate dental connection outsourcing- including product basics, outsourcing potential, manufacturing benefits in China, workflow management, cost reasoning, quality regimes, and risk responses. Due to its straightforward and easily comprehensible language, the article is intended to give you, regardless of your position as a laboratory owner, a procurement manager, or a clinical decision-maker, an opportunity to make uncomplicated decisions about outsourcing that are neither very risky.
1. What Is a Precision Dental Attachment?
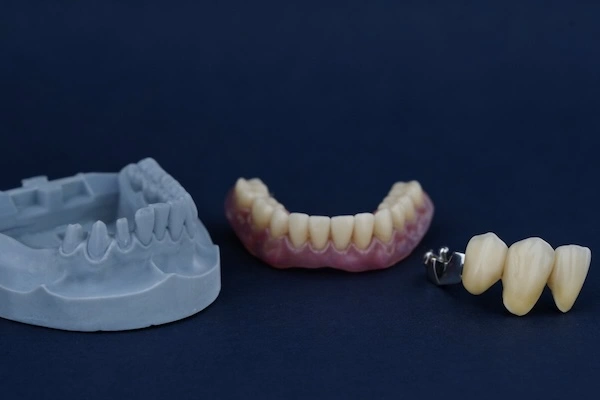
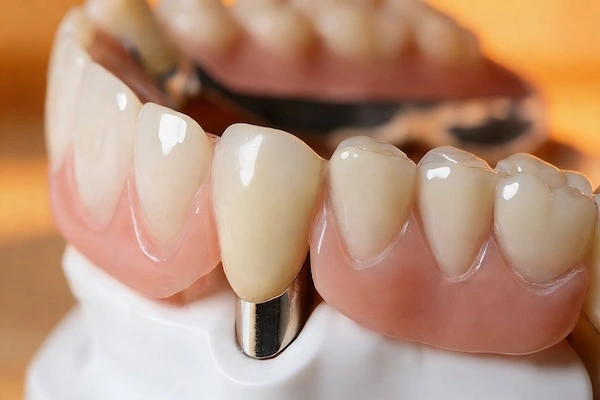
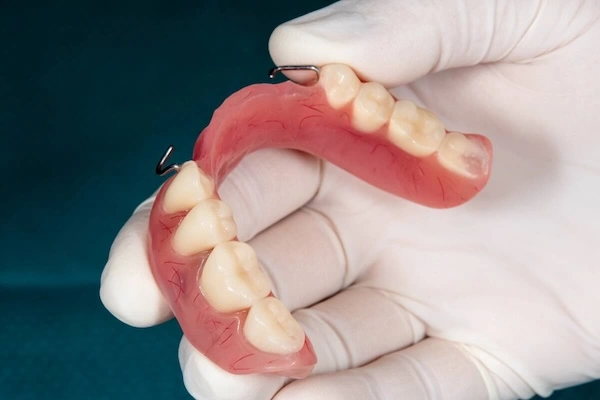
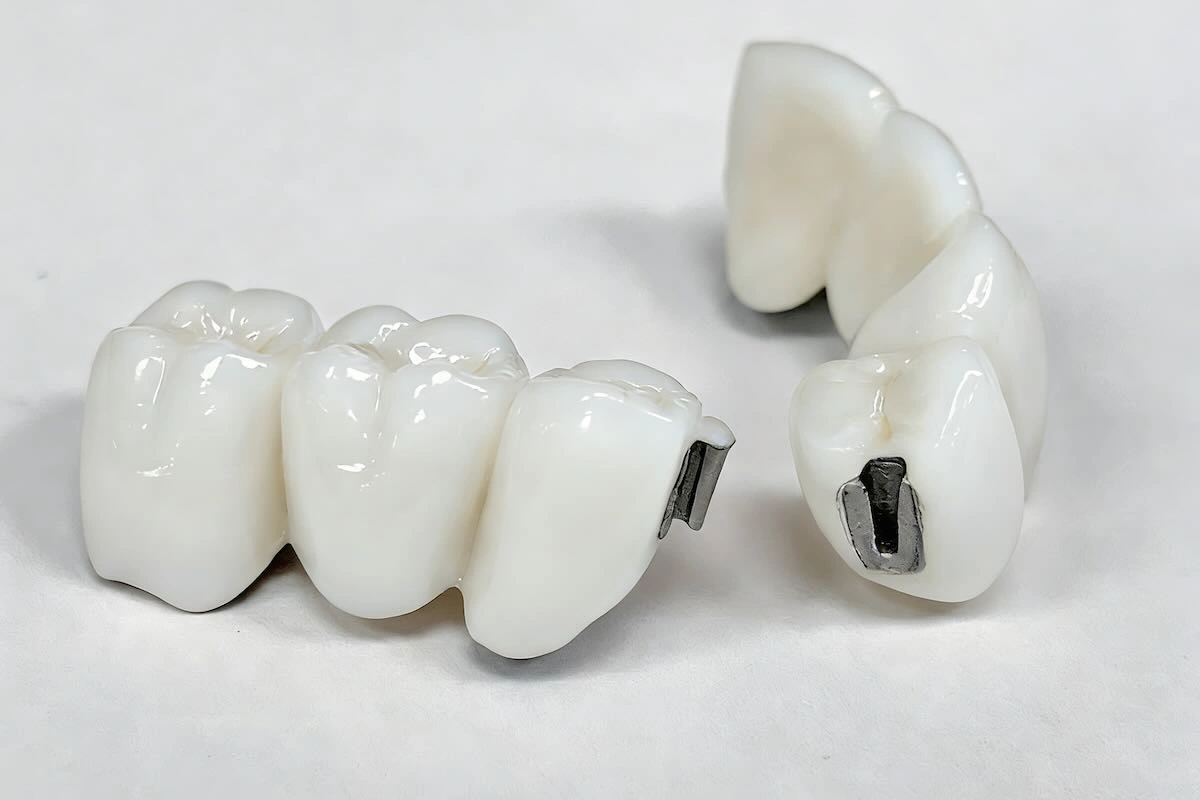
Precision dental attachments are mechanical holding elements applied to mostly removable partial dentures (RPDs) and implant-assisted removable restorations. As opposed to traditional types of clasps, which are visible and are based on flexible metal noodles, precision attachments are the invisible types of retention heads and are aimed at enhancing the aesthetic, comfort, and functional performance.
Structurally, a precision attachment is usually a combination of two parts:
- Male part (patrix): Typically formed as a part of a crown, bridge, or abutment to an implant
- Female part (matrix): It is embedded in the removable denture structure
With restrictions, these two parts interact with the controlled friction or mechanical locking, and hence the denture becomes removable and at the same time maintains a high level of retention and stability.
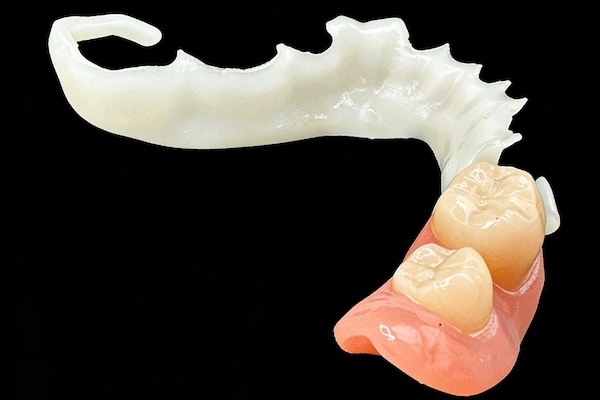
Common Types of Precision Attachments
- Extracoronal attachments: These are located external to the contour of the crown, and are frequently found in distal extension cases
- Intracoronal attachments: Entirely embedded in the crown, which is the most attractive
- Stud attachments: Frequently used with implants (e.g., ball, locator-type systems)
- Bar attachments: Splinted implant or tooth-supported overdentures
To the non-experts, the precision attachments can be defined as invisible connections that substitute the visible metal attachments but enhance comfort and wearability in the long run.
2. Why Are Precision Dental Attachments Suitable for Outsourcing?
Dental attachments, which are particularly precise, are especially fitting to outsource because of their repeatable manufacturing logic, high intensity of labor, and good compatibility with digital workflows. Their manufacturing can be readily done in centralized locations since their production is based on standardized technical execution as opposed to chairside decision-making.
(1) Agreement on high technical labor requirements
Attachment fabrication entails detailed milling/casting, detailed fitting, and detailed polishing. These labor-intensive processes make production costs much higher in high-wage areas, and, therefore, outsourcing is a structurally efficient answer to these challenges.
(2) Standardized Processes of Manufacturing
After the verification of design parameters, the process of attachment production is repeatable and adheres to the same pattern of work. The level of standardization enables remote manufacturing to have consistent levels of quality with little variation.
(3) Online Compatibility in Design
The CAD/CAM design files, STL data, and scanned models allow transfer of the case as well as collaborative work remotely and promote foreseeable production across the border without physical presence.
(4) Clear Quality Benchmarks
It is the nature of precision attachments that their tolerances and functional requirements are well-defined so that quality control can be systematic and measurable, as well as auditable.
Consequently, there is no uncertainty created with outsourcing; since the manufacturing process is labor-intensive, it is transferred over to more specialized providers, as the client still maintains control of the design and the clinical decision-making.
3. Why Outsource Precision Dental Attachments to China?
China has emerged as a world production hub in dental prosthetic production, especially in advanced removable products. The cost factor is not the main reason behind the decision to outsource to China, but it is a set of scale, depth of skills, digital sophistication, and integration of supply chains.
- Talented Technological Labor Force: Chinese dental technicians usually work in the field of removable prosthetics and attachments and have years of practical experience in international standards.
- Mature Dental CAD/CAM Infrastructure: Precision attachment production on scale is aided by advanced milling centers, laser sintering, CNC machining, and digital design teams.
- Efficiency in Cost by Structure, not Compromise: The reduced cost of labor and operation enables affordable prices to be charged without compromising the quality of materials or process control.
- High Export Familiarity: Top Chinese laboratories are used to FDA, CE, and other international standards compliance, and English-speaking project coordinators.
- Scalable Capacity: Pilot cases and mass production can be managed in China-based laboratories without volatility in lead time.
To foreign clients, China is a middle ground of cheapness, trustworthiness, and technical expertise.
4. What Can and Cannot Be Outsourced?
It is also important to establish the scope of outsourcing properly to establish the right expectations and prevent remakes. The separation of manufacturing roles and clinical decision-making assists both parties to operate in their areas of strength, limits the number of communication errors, and provides the same results in all the outsourced cases.
1) What Can Be Outsourced
- Precision attachment fabrication (metal or polymer components)
- Attachment case CAD design
- Casting or milling of attachment frameworks
- Integration of attachment into the RPD frameworks
- Implant-supported attachment elements
- Final and try-in prosthesis production
- Finishing, polishing, and assembly
2) What Should Typically Remain Local
- Final clinical adjustment
- One-sided fitting and fine-tuning of the occlusals
- Emergency and immediate relines
- Cases that need to be handled within a day
Outsourcing works optimally when manufacturing operations that are complex and labor-intensive are concentrated in the specialized production plants, and case-specific clinical judgment and final modifications are made locally. This line of responsibility assists with maintaining clinical control, increasing efficiency of production, and reducing the chances of delays and unwarranted remakes.
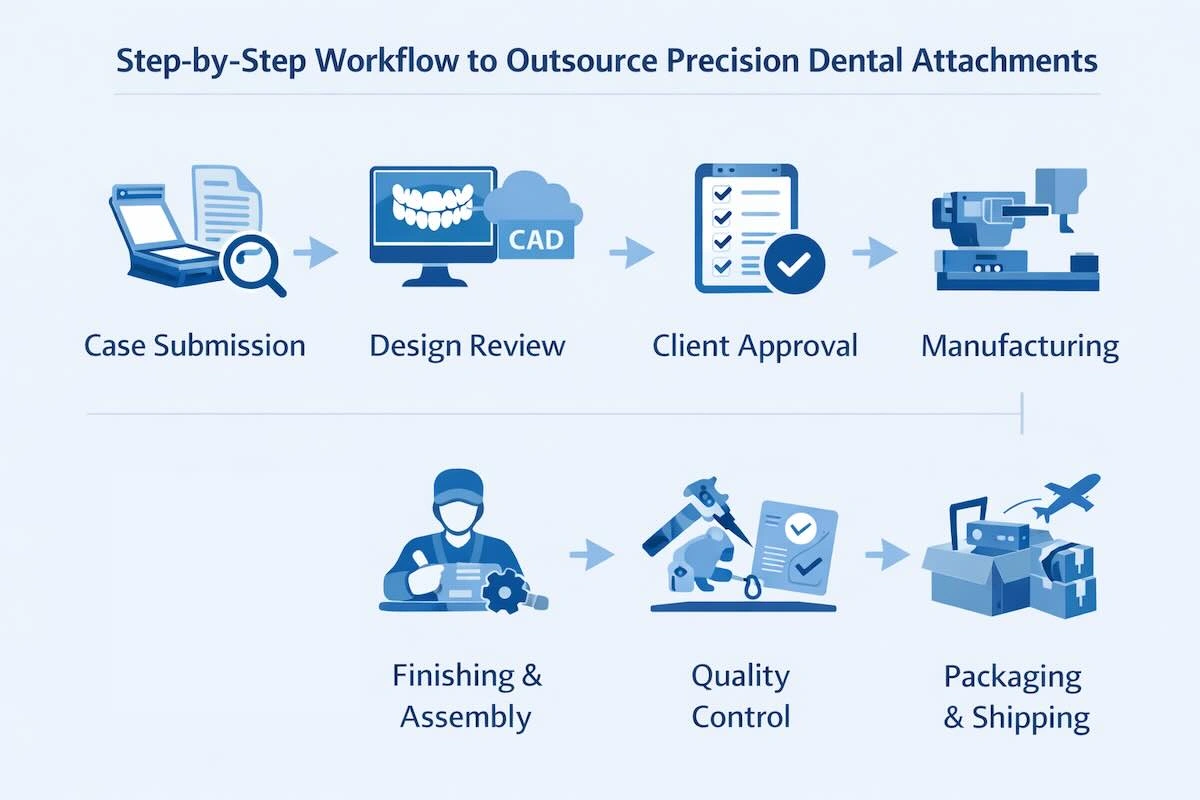
5. Step-by-Step Workflow to Outsource Precision Dental Attachments
The most important aspect of successful precision attachment outsourcing is a controlled and transparent workflow. Since these restorations have close tolerances and working interlocking parts, each of the steps, including case reception up to finished delivery, is to be well defined, analyzed, and recorded. A professional Chinese dental laboratory will thus be subject to a structured and audit process that is aimed at reducing ambiguity and risk of remake.
Step 1: Case Submission
Proper and comprehensive input of the case also initiates the outsourcing process. Initial clear information is paramount because any missing or vague information at this point will be increased in the later stages of production.
- The main aspects that should be established upon submission:
- Electronic replication or physical impressions with distinct margins and places of attachment.
- Specific prescription describing the type of attachment, the strength of retention, and the material.
- Reference photos, occlusal records, and opposing models were necessary.
The upfront provision of all case information provides a good background of reliable design and production.
Step 2: Design Review
The laboratory will carry out a design review to check the feasibility, the position of the attachments, and the path of insertion once the case has been received. This action converts clinical intent into the digital geometry to be manufactured.
In the process of design review, the lab will generally:
- Design a CAD in terms of attachment placement and the structure of the framework
- Examine undercuts, paths of insertion, and space
- Determine the risks that may occur and report design information to the client
It is a team stage that is, by nature, the main technical gateway to production commitment.
Step 3: Client Approval
The client has to consider and approve the suggested design before any actual manufacturing takes place. This step of approval is mandatory in preserving design authority on the client side.
Approval usually includes:
- Review STL files, screenshots, or design snapshots
- Hypothesis validation of attachment-type, positioning, and retention concept
- Formal consent to go ahead with the production
Formal approval assists in avoiding downstream conflicts, and the responsibility areas are well outlined.
Step 4: Manufacturing
The case is then approved, and it goes through the manufacturing process, whereby the digital designs are converted into the physical components through the application of the relevant production procedures.
Examples of manufacturing processes are:
- Depending on the material choice, CNC milling, casting, or laser sintering may be done
- Careful printing of the components of the attachment
- Attachment incorporation into RPD or implant-supported structures
Process control and experience of technicians are at this point very important to acquire a proper fit and functional engagement.
Step 5: Finishing & Assembly
After the primary fabrication, the case continues to the finishing and assembly, where the hand in work is of great importance in the final performance.
The steps to this usually include:
- Attention to the manual polishing of attachment surfaces
- Male and female component assembly and engagement test
- Functional simulation to determine retention and insertion behavior
They are finished correctly and therefore are very smooth to operate, comfortable to the patient, and do not wear out easily.
Step 6: Quality Control
The process of quality control is performed at a devoted level and not as a post-factum. In the case of precision attachments, QC is aimed at dimensional accuracy as well as functional reliability.
QC checks commonly include:
- Dimensional checking with design requirements
- Retention force and engagement test
- Functional and visual examination of the assembly was completed
Multi-point QC is associated with a substantial decrease in the probability of clinical adjustment problems.
Step 7: Packaging & Shipping
After the case has been cleared in quality, it is ready to be shipped internationally, considering the issue of protection and traceability.
Final preparation includes:
- Tamperproof packaging to avoid deformation or breakage
- Export and shipping papers done
- Tracking of shipments and delivery arrangements
The case is well packaged, and its logistics are well managed, such that the case is ready to be used clinically.
This sequential flow of work gives foresight, visibility, and responsibility in the outsourcing process, and the dental labs and clinics can sustain control and enjoy the benefit of special manufacturing offshore.
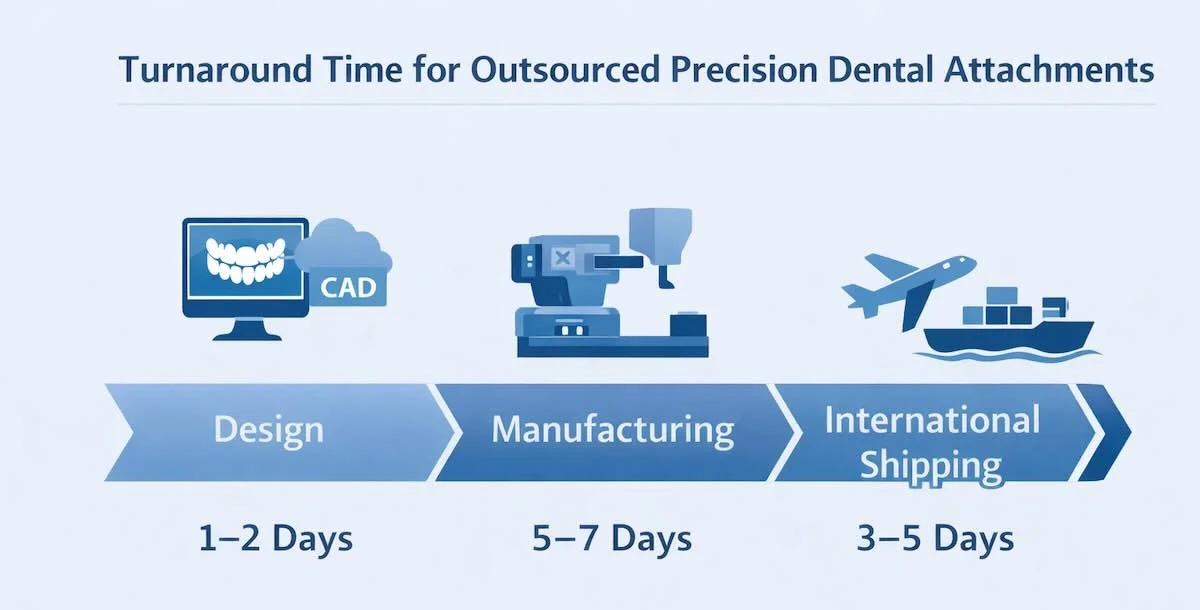
6. Turnaround Time for Outsourced Precision Dental Attachments
Turnaround time is among the most vital factors for international labs and clinics in outsourcing the precision dental attachments. Though precise schedules will also differ based on the complexity of the cases, type of attachment, and logistic provisions, established Chinese dental laboratories can always fall into fixed schedule production schedules that can be used to deliver their products predictably and reliably.
In normal circumstances, the common turnaround standards are as follows:
(1) Design phase: 1-2 working days
This phase entails case analysis, CAD, and internal feasibility analysis. Good prescriptions and all-digital information also hugely reduce this process.
(2) Production and QC: 5-7 working days
Attachment fabrication, framework production, finishing, assembly, and multi-stage quality inspections fall within this period. The other, more complicated cases or special materials can take longer.
(3) International shipping: 3-5 working days
The duration of delivery varies based on the country where the goods will be delivered, the clearing procedures in the country, and the level of courier service. The majority of the laboratories employ trackable express logistics for clients outside the country.
(4) Total average turnaround time: 9-14 working days
It should be mentioned that not only the laboratory capacity but also the quality of communication and the efficiency of the approval at the client side will be important to achieve the stability of turnaround time. Late confirmation of design or incomplete case information are some of the frequent culprits of the long timelines.
Special rush services can be offered on individual cases, but should be applied to clearly-defined, low-risk restorations. Constant planning and a standard workflow, in most cases, offer greater overall reliability as opposed to last-minute acceleration.
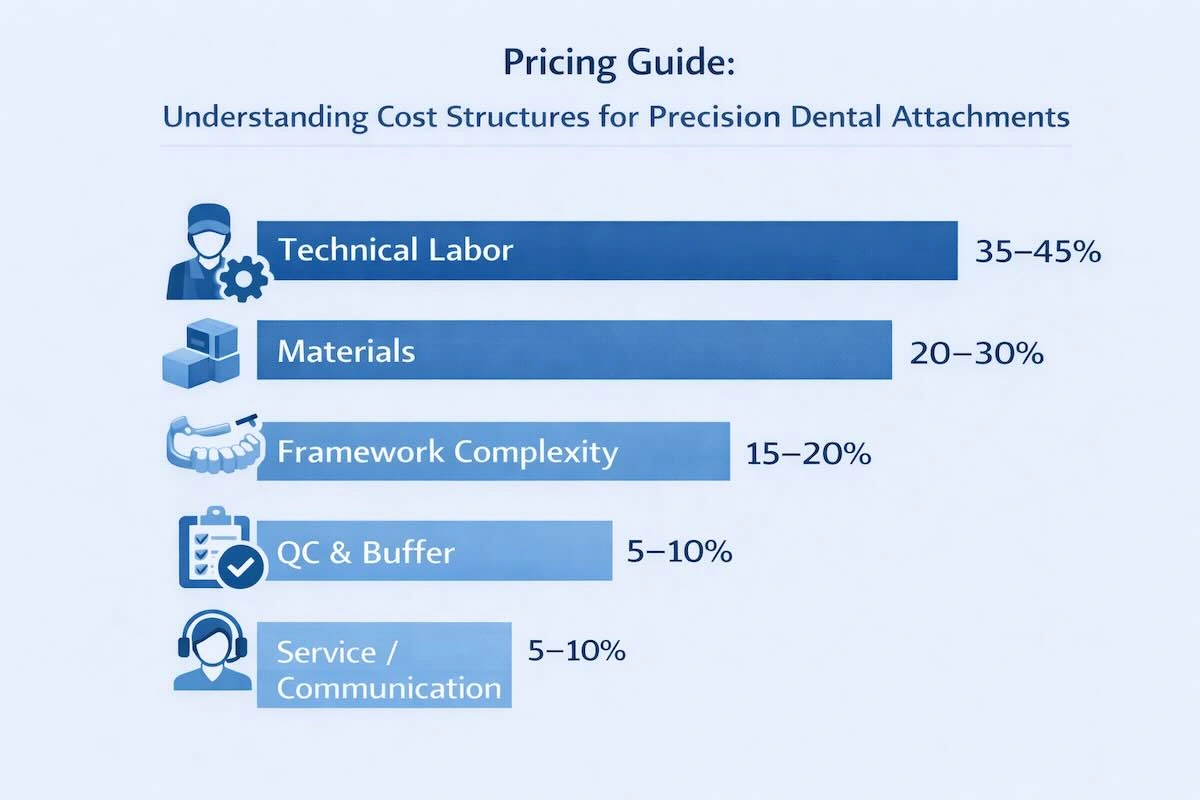
7. Pricing Guide: Understanding Cost Structures for Precision Dental Attachments
The precision dental attachment price is set based on a mixture of the input of technical labor, the material used, and the complexity of the case, and not on a unit cost. To international customers, it is more important to know how these aspects are added to the ultimate quotation rather than just comparing prices because they aid in differentiating structural cost effectiveness and quality-based trade-offs.
| Cost Factor | Typical Impact | Practical Explanation |
| Technical labor | 35–45% | CAD design, attachment fitting, manual finishing, and assembly |
| Material selection | 20–30% | Co-Cr, titanium, or precious alloys with different processing demands |
| Framework complexity | 15–20% | Number of attachments, insertion path, and spatial constraints |
| QC and rework buffer | 5–10% | Multi-stage inspection, tolerance control, and remake risk |
| Communication & service | 5–10% | Case coordination, design review, and technical support |
Although Chinese dental laboratories usually have a cost advantage of 30-50 percent, instead of saving on materials or reducing quality control, lower labor costs, centralized production, and high operational efficiency are often the contributors to the advantage. This is why the prices should be evaluated with constant regard to the policies of remake, the scope of QC, and communication support, because the lowest price offered is not always the lowest cost in the end.
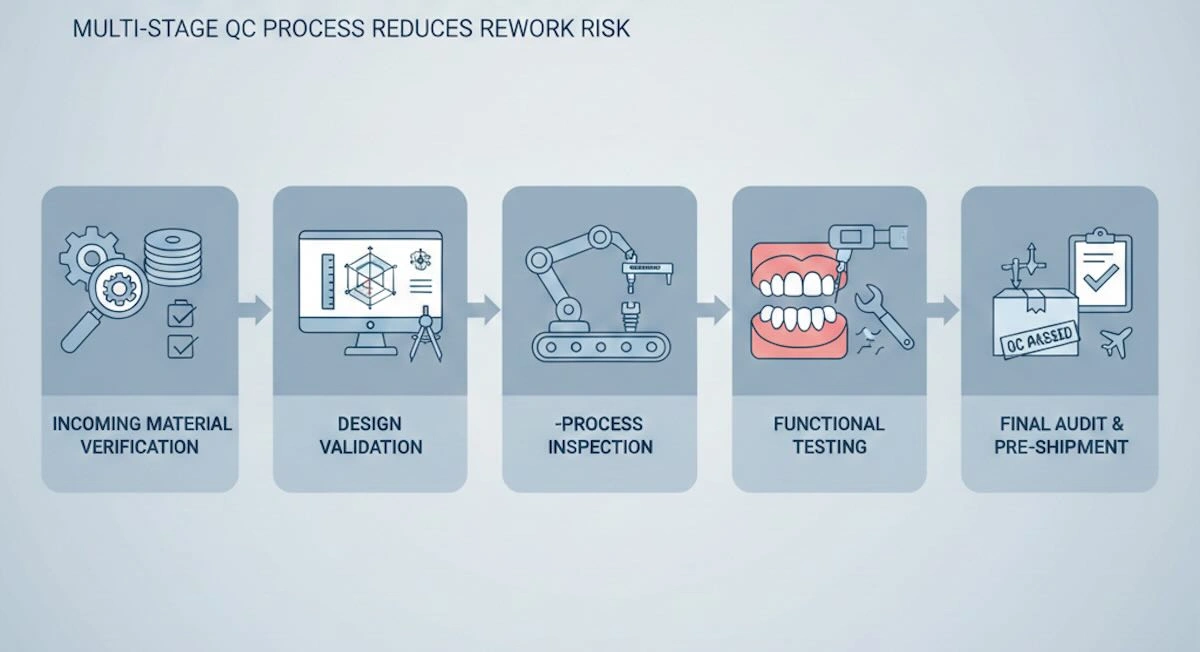
8. Quality Control Systems for Outsourced Precision Dental Attachments
Successful outsourcing is based on quality control, whereby the precision dental attachments will be delivered to the client in a quality manner that will satisfy the functional as well as the dimensional needs. Instead of completing the inspection only at the final stage, a strong QC system will monitor the quality during the whole production process.
(1) Inbound Material Verification
Compliance with specifications is confirmed on all raw materials before the commencement of production to avoid errors as early as possible, before production starts, and before the metal alloys or polymer components are used.
(2) Design Validation
CAD designs are checked in terms of their feasibility, the correct location of attachments, and tolerance, such that the clinical intent may be correctly implemented in the production process.
(3) In-Process Inspection
Dimensional checks and alignment verification are performed during the milling, casting, or assembly to identify deviations at an early stage and minimize remakes.
(4) Functional Testing
Attachment retention, engagement, and easy insertion/removal also get tested to ensure that there is a match between performance and the requirements of the prescription.
(5) Final Check and Audit at Pre-Shipment
Finished goods are subject to visual and mechanical inspection, examination of documentation, and verification of packaging to ensure that all the deliveries are of high quality and are prepared to be shipped.
Throughout the process, professional laboratories will be able to identify and rectify the problems at the early stages, reducing clinical corrections and increasing the level of confidence in outsourcing.
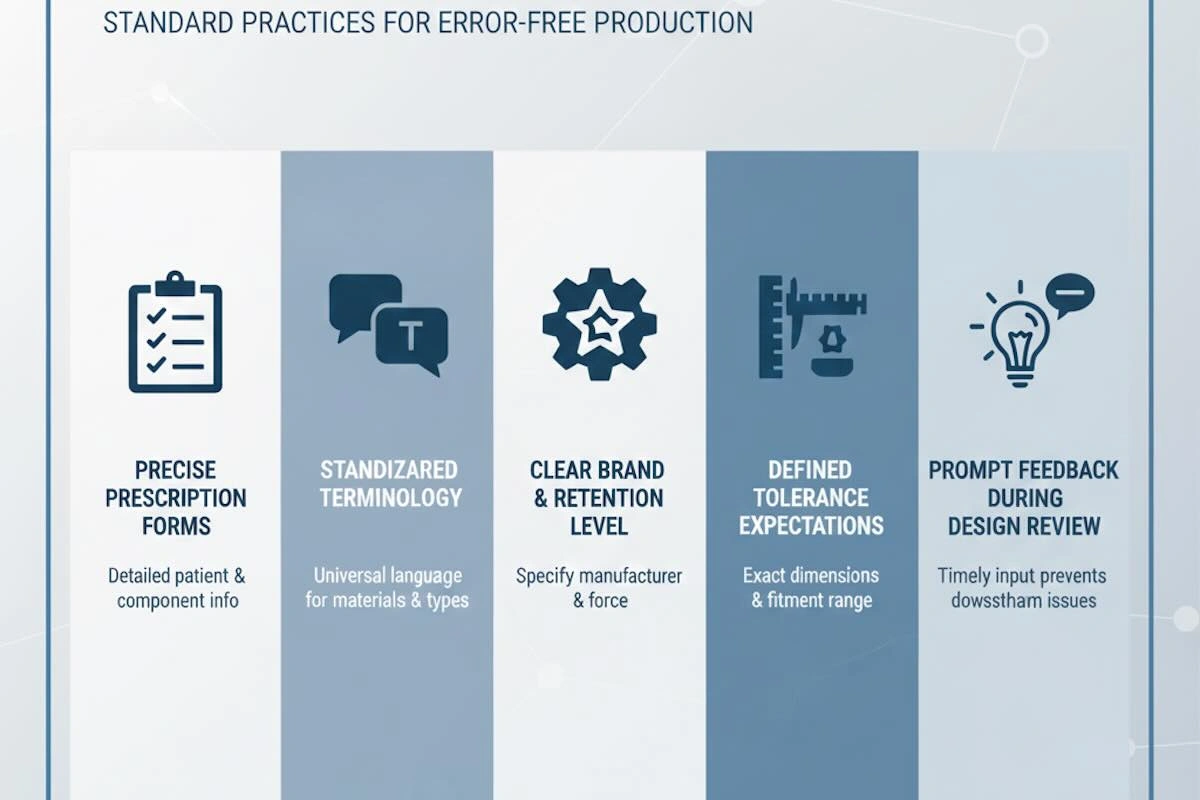
9. Communication Requirements to Reduce Remakes in Attachment Outsourcing
Effective and well-organized communication has a finalizing aspect in reducing remakes in outsourcing precision dental attachments. Attachment performance is a key element that relies on design intent and tolerance control; any minor misunderstanding will result in functional inconsistencies.
To minimize the risk of remake, clients must:
- Accurate and full prescription forms for individual cases
- Uniform, standardized communication terminology
- Evidence of attachment brand, system, and retention levels
- Clear tolerance expectations and effective priorities
- Timely and integrated feedback on the design review stage
Specialized case coordinators are used in effectively managed outsourcing relationships where the clinical intent and technical implementation are properly translated and kept on track during the workflow.
10. Risks and Mitigation Strategies in Precision Dental Attachment Outsourcing
Outsourcing of precision dental attachments is a gamut of risks, such as technical deviations and communication delays. Knowing of possible problems and applying proactive mitigation will be the key to keeping quality, reducing remakes, and having quality predictability in delivery.
| Risk | Cause | Mitigation Measure |
| Misinterpretation of design intent | Incomplete or unclear prescriptions, ambiguous CAD files | Pilot cases, detailed case instructions, and early client review |
| Inadequate retention strength | Incorrect attachment selection or fabrication tolerances | Multi-stage functional testing and retention verification |
| Fit discrepancies | Dimensional inaccuracies during milling, casting, or assembly | In-process dimensional checks and design validation |
| Delayed communication | Slow feedback or approval from the client | Clear approval checkpoints and a dedicated case coordinator |
| Remake uncertainty | Ambiguous responsibility for adjustments | Written remake policies and a formal agreement of responsibilities |
| Production scaling issues | Large-volume orders without phased testing | Start with pilot cases and stable long-term partnerships |
Managing these risks is not about their full removal, which is hardly ever possible, but rather about control, predictability, and organization. Defining the most frequent failure points and matching them with individual mitigation strategies would help labs and clinics to outsource the precision dental attachments comfortably and without any decline in clinical standards and operational efficiency.
11. How to Choose the Right Precision Attachment Supplier
The need to have a good supplier is important to the success of the outsourcing of precision dental attachments. In addition to the pricing competition, international laboratories and clinics must consider the possible partners according to experience, technical competence, quality systems, and communication practice because they have a direct impact on the fit, functionality, and turnaround reliability.
The main criteria to be considered are:
- Existing experience in removable prosthetics: The suppliers must possess experience in creating precision attachments to partial dentures and implant-supported restorations.
- Digital design capability: Ability to have CAD/CAM files, STL data, and digital workflows processed effectively.
- Clear quality control measures: Multi-phase QC procedures, which are verifiable and auditable.
- English-speaking technical support: To minimize remakes, fluidity of communication is necessary in order to have clarity of prescription.
- Constant production capacity: The lab should not experience any delays in dealing with pilot cases and large volume orders.
- Efficient accountability system: Responsibilities for design approval, remakes, and quality issues are defined.
For foreign customers in need of a company that has all these attributes, Bestodental will be a long-term expansion, which is reliable. Having a long history of removable prosthetics production, a complete digital workflow, systematic QC procedures, and specially trained English-speaking case managers, Bestodental not only offers a manufacturing service but a strategic outsourcing joint venture, where the quality is predictable, delivery is timely, and there is effective communication. It is therefore a perfect selection when used in laboratories and clinics that would want to increase the volume of their products without compromising clinical control.

12. Who Should and Should Not Outsource Precision Dental Attachments?
Precision dental attachment outsourcing is not an absolutely perfect decision. The knowledge of the laboratories/clinics that are the most beneficial and those that are likely to experience difficulties contributes to setting realistic expectations and facilitates proper cooperation.
1) Suitable for Outsourcing
- Laboratories with high volumes: Have a chance to use labor outsourcing to cut labor expenses and produce on a larger scale.
- Clinics that have predictable case flow: Case volumes are stable and can be scheduled and assured turnaround.
- Digital workflow consumers: CAD/CAM and STL-approachable practices can transfer designs without errors or remakes.
- Markets where cost matters: Outsourcing will likely save considerable money without reducing quality in high-wage areas.
2) Less Suitable for Outsourcing
- Urgent practices: Cases that have to be completed within a day or in a hurry are hard to predict and rely on.
- Extremely low-volume cases: The sporadic orders might not warrant setting up and may add more to per-unit cost.
- Clinics that do not use data capture digitally: Loss of precise digital impressions/scans increases the complexity of communication and risk of remake.
Laboratories and clinics can make wise decisions regarding outsourcing by making a careful evaluation of suitability. In the right partnership, outsourcing enhances operational performance, saves on cost, and ensures clinical care, but a lack of alignment on volume, workflow, or urgency will cause delays, remakes, and frustration. The identification of these boundaries will make outsourcing a strategic benefit and not a risk factor.
13. Precision Dental Attachment Outsourcing FAQs
The outsourcing of precision dental attachments is often subject to certain questions by the laboratories and clinics. The questions and answers below are practical, technical, and operational questions that are usually associated with cross-border collaboration to assist decision-makers in assessing risks, working processes, and service expectations.
Q1. Can outsourcing precision attachments be safe in complicated or sophisticated cases of prosthetics?
Yes. Even those cases that involve tight tolerances or brand-specific components may be outsourced safely when a laboratory adheres to the multi-stage QC, functional, and structured communication.
Q2. Is the outsourcing of brand-specific or proprietary attachment systems possible?
The vast majority of mainstream attachment systems are compatible, although they need to be confirmed. Suppliers are supposed to check design files, tolerances, and insertion paths prior to contracting to manufacture.
Q3. What does outsourcing workflow do with remakes and adjustments?
Professional labs also have definite remake policies that delineate the areas of responsibility between the client and manufacturer. Pilot cases, early approval, and documented QC greatly decrease the rate of remakes.
Q4. What is the level of protection of intellectual property (IP) and sensitive patient data?
Reliable suppliers have stringent confidentiality rules, file transfer systems, and limited entry to computer scans and CAD records in order to protect IP and patient data.
Q5. What are the most effective factors that determine the turnaround time and reliability in delivery?
Delivery is influenced by case complexity, speed of approval, selection of materials, and the logistics of shipping materials internationally. Consistent turnaround is heavily dependent on predictable scheduling, total digital data, and consistent communication channels.
14. Conclusion
Subcontracting of precision dental attachments to relevant laboratories in China provides laboratories and clinics with a sure mechanism to save costs, enhance efficiency, and maintain high-quality standards. Through systematized workflows, multi-phase quality management, and online partnership, global clients will be able to attain consistent results whilst retaining design control and clinical decision-making internally.
Bestodental is a good choice that integrates technical knowledge, open operations, and commitment to clients to ensure precision attachment outsourcing is smooth sailing and manageable to those who want to have a long-term and reliable partner. The cooperation with such a laboratory as Bestodental guarantees the high quality and time-scheduling, not only that, but also a belief that it is possible to scale production without neglecting the clinical standards.